

What is even more remarkable is that those 600 amino acids are encoded by three nucleotides each, and the mutation is caused by a single base change (point mutation), 1 in 1800 bases.įigure 3. The structural difference between a normal hemoglobin molecule and a sickle cell molecule-which dramatically decreases life expectancy-is a single amino acid of the 600. The molecule, therefore, has about 600 amino acids. What is most remarkable to consider is that a hemoglobin molecule is made up of two alpha chains and two beta chains that each consist of about 150 amino acids. Specifically, the amino acid glutamic acid is substituted by valine in the β chain. In sickle cell anemia, the hemoglobin β chain (a small portion of which is shown in Figure 2) has a single amino acid substitution, causing a change in protein structure and function. A change in nucleotide sequence of the gene’s coding region may lead to a different amino acid being added to the growing polypeptide chain, causing a change in protein structure and function. The unique sequence for every protein is ultimately determined by the gene encoding the protein. In sickle cell hemoglobin, this glutamate is replaced by a valine.
In normal hemoglobin, the amino acid at position seven is glutamate. The beta chain of hemoglobin is 147 residues in length, yet a single amino acid substitution leads to sickle cell anemia.
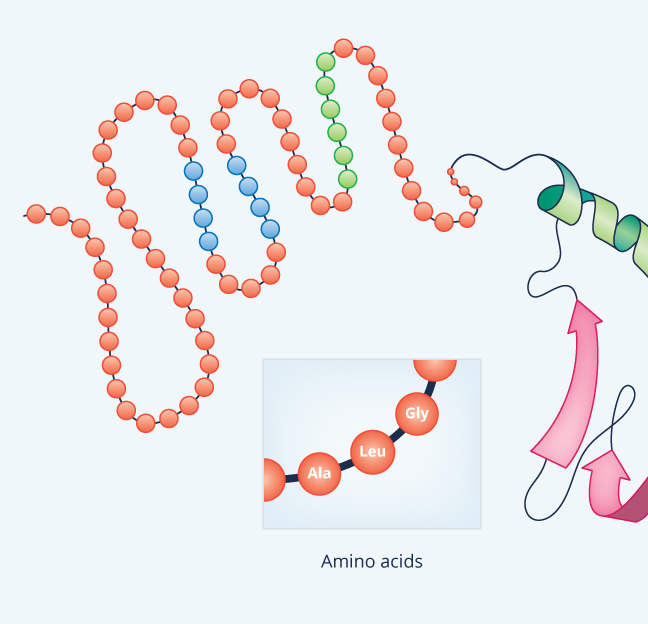
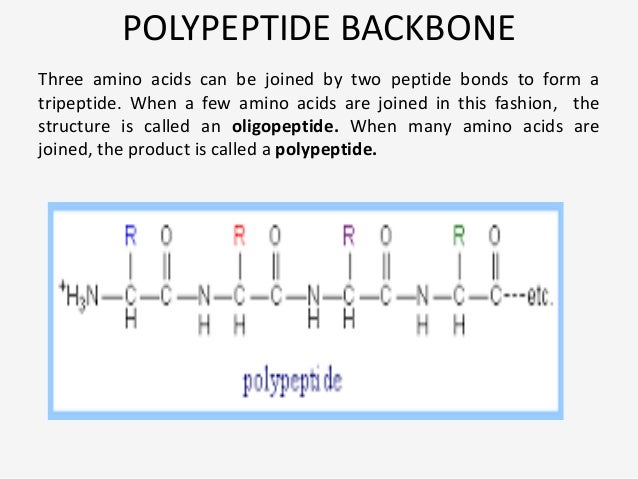
Variants of ZBTB7A (LRF) and its β-globin gene cluster binding motifs in sickle cell anemia. Shaikho EM, Habara AH, Alsultan A, Al-Rubaish AM, Al-Muhanna F, Naserullah Z, Alsuliman A, Qutub HO, Patra PK, Sebastiani P, Baltrusaitis K, Farrell JJ, Jiang Z, Luo HY, Chui DH, Al-Ali AK, Steinberg MH. Secondary and Supersecondary Structure of Proteins in Light of the Structure of Hydrophobic Cores. Blind tests of RNA-protein binding affinity prediction. Kappel K, Jarmoskaite I, Vaidyanathan PP, Greenleaf WJ, Herschlag D, Das R. Drug-induced activation of integrin alpha IIb beta 3 leads to minor localized structural changes. Janke U, Kulke M, Buchholz I, Geist N, Langel W, Delcea M. Quaternary structure is the association between two or more polypeptides, but not every protein has a quaternary structure.Ĭopyright © 2023, StatPearls Publishing LLC. Tertiary structure is the three-dimensional shape of the protein determined by regions stabilized by interactions between the side chains. Secondary structure is comprised of regions stabilized by hydrogen bonds between atoms in the polypeptide backbone. In brief, primary structure is the linear chain of amino acids. By forming peptide bonds between the amino and carboxyl groups on two different amino acids, large polypeptide chains can be created.Įvery protein can be described according to its primary structure, secondary structure, tertiary structure, and quaternary structure. As diverse as they can be, they are all made up of the same 20 amino acids. They are known as the most structurally complicated biological molecules. Each of these proteins has its own structure and function. Humans have tens of thousands of proteins in their bodies at any given moment in time. Different proteins can play a role in speeding up chemical reactions, storage, defense, cell communication, movement, and structural support. There are many different types of proteins. They account for 50% of the dry mass of cells and play a role in everything an organism does. Nearly every function in living beings depends on proteins.


 0 kommentar(er)
0 kommentar(er)
